Multiple Choice
Identify the
choice that best completes the statement or answers the question.
|
|
|
1.
|
Which atom in the reaction below has a decrease of
3 in oxidation number?
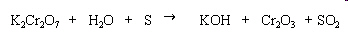
|
|
|
2.
|
If the half reaction below is balanced in acidic
solution, how many moles of hydrogen
ions are required to balance the overall
equation?
|
|
|
3.
|
What is the oxidation number of carbon in
CO32-?
|
|
|
4.
|
Which is reduced in the reaction
below?
a. | O2(g) | b. | O2-(aq) | c. | Sn(s) | d. | Sn2+(aq) |
|
|
|
5.
|
Which half reaction is balanced for both atoms and
charge?
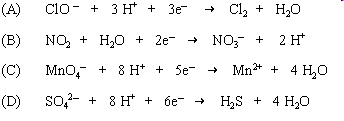
|
|
|
6.
|
What is the Eo
value for the reduction of Y3+(aq) in the reaction
below?
a. | -2.37 | b. | -1.66 | c. | +1.66 | d. | +2.37 |
|
|
|
7.
|
Which is the cathode in the diagram
below?
a. | Cu(s) | b. | Cu2+(aq) | c. | Pb(s) | d. | Pb2+(aq) |
|
|
|
8.
|
What is the oxidation number of O in O2(g)?
|
|
|
9.
|
Which species is reduced in the reaction
below?
a. | Zn(s) | b. | Zn2+(aq) | c. | Cu(s) | d. | Cu2+(aq) |
|
|
|
10.
|
How are pyrometallurgy and hydrometallurgy
similar?
a. | They are primary treatments that involve extracting
metals. | b. | They are primary treatments that involve refining
metals. | c. | They are secondary treatments that involve extracting
metals. | d. | They are secondary treatments that involve refining
metals. |
|