Multiple Choice
Identify the
choice that best completes the statement or answers the question.
|
|
|
1.
|
How is the molar enthalpy change calculated for a
chemical reaction?
a. | SPEproducts - SPEreactants | b. | SPEproducts
+ SPEreactants | c. | SPEreactants- SPEproducts | d. | SPEreactants + SPEproducts |
|
|
|
2.
|
What is DHocomb of propanol, C3H7OH,
if burning 10.0 g of propanol releases 336 kJ of
energy?
a. | -2020 kJ/mol | b. | -55.9 kJ/mol | c. | -33.6
kJ/mol | d. | -0.0298 kJ/mol |
|
|
|
3.
|
What is the energy required to raise the
temperature of 1.0 g of a substance by 1.0 oC?
a. | fuel value | b. | heat capacity | c. | one
joule | d. | specific heat
capacity |
|
|
|
4.
|
Which involves the greatest energy
change?
a. | (s) --> (l) | b. | (l) --> (s) | c. | (s) -->
(g) | d. | (l) --> (g) |
|
|
|
5.
|
What is the molar enthalpy change for the reaction
below?
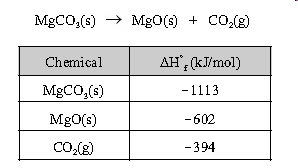
a. | -2109 kJ/mol | b. | -117
kJ/mol | c. | 117 kJ/mol | d. | 2109 kJ/mol |
|
|
|
6.
|
Which energy change occurs when water is heated
from 37.0 oC to 95.0 oC?
a. | kinetic energy decreases | b. | kinetic energy increases | c. | potential energy
decreases | d. | potential energy
increases |
|
|
|
7.
|
Which is the correct unit for heat
capacity?
|
|
|
8.
|
How much energy is contained in a 50.0 g cereal bar
if its fuel value is 0.0134 kJ/g?
a. | 2.68 x 10-4 kJ | b. | 1.34 x 10-2 kJ | c. | 6.70 x
10-1 kJ | d. | 3.73 x
103 kJ |
|
|
|
9.
|
Which is true for an exothermic
reaction?
a. | enthalpy difference between products and reactants
(DH) is negative | b. | enthalpy
difference between products and reactants (DH) is
positive | c. | enthalpy of the products is higher than the enthalpy of
the reactants | d. | enthalpy of the
products is the same as the enthalpy of the reactants |
|
|
|
10.
|
At standard pressure, which is an example of a
change in kinetic energy only?
a. | carbon dioxide cooling from -80oC to -100oC | b. | molten aluminum
solidifying at 600oC | c. | steam condensing at
100oC | d. | water decomposing above 1.0 x 107
oC |
|