Multiple Choice
Identify the
choice that best completes the statement or answers the question.
|
|
|
1.
|
Which equation represents the reaction of a
Brønsted-Lowry base with water?
|
|
|
2.
|
At a given temperature a sample of pure water has a
pH = 7.10. Which of the following is true?
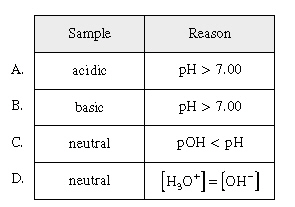
|
|
|
3.
|
Consider the following indicator
equilibrium:
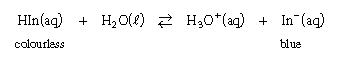 What is
the effect of adding HCl to a blue sample of this indicator? What is
the effect of adding HCl to a blue sample of this indicator?
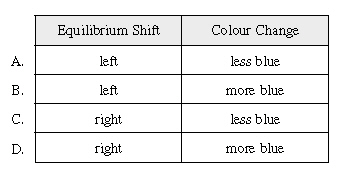
|
|
|
4.
|
Oxalic acid dihydrate is a pure, stable,
crystalline substance. Which of the following
describes one of its uses in acid-base
titrations?
a. | buffer | c. | chemical indicator | b. | primary
standard | d. | stoichiometric indicator |
|
|
|
5.
|
Which of the following would be used to prepare an
acidic buffer solution?
a. | HF and H3O+ | c. | NH3
and NH4Cl | b. | H2S and NaHS | d. | HNO3 and NaNO3 |
|
|
|
6.
|
Which acid forms a 0.10 mol/L solution with the
highest pH?
a. | acetic acid | c. | oxalic
acid | b. | nitrous acid | d. | phosphoric
acid |
|
|
|
7.
|
What is the pH of a solution if [H3O+] is 0.0001 mol/L?
|
|
|
8.
|
Which is a monoprotic acid?
a. | HCOOH(aq) | c. | H3BO3(aq) | b. | H2CO3(aq) | d. | NaOH(aq) |
|
|
|
9.
|
Which best describes a 10.0 mol/L solution of
hydrofluoric acid?
a. | strong and concentrated | c. | weak and concentrated | b. | strong and
dilute | d. | weak and dilute |
|
|
|
10.
|
A flask containing an unknown solution of
concentration 0.100 mol/L, is tested with three
indicators. Based on the data below, what is the
pH of this solution?
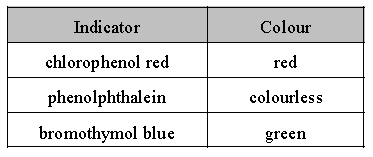
|