Multiple Choice
Identify the
choice that best completes the statement or answers the question.
|
|
|
1.
|
Consider the following diagram for the equilibrium
system:
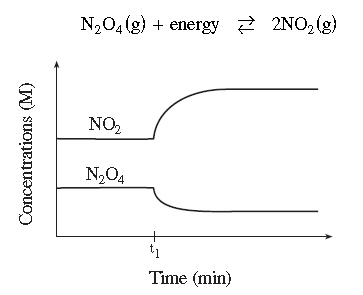 Which of the following stresses was applied at
time t 1?
a. | [NO2] was increased | c. | Temperature was
increased | b. | [N2O4] was decreased | d. | Temperature was
decreased |
|
|
|
2.
|
Consider the following reaction:
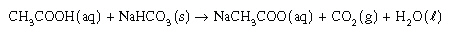 Which of the following properties could best be used
to measure the reaction rate? Which of the following properties could best be used
to measure the reaction rate?
a. | the volume of CO2 | c. | the mass of
CH3COOH | b. | the volume of
H2O | d. | the surface
area of NaHCO3 |
|
|
|
3.
|
Consider the reaction:
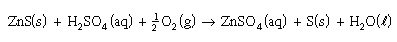 What would increase the change of successful
collisions? What would increase the change of successful
collisions?

a. | I and II only | c. | II and III only | b. | I and IV only | d. | I, II, III and
IV |
|
|
|
4.
|
Consider the following two reactions occurring
under the same conditions:
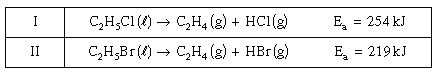 Which of
the following is correct? Which of
the following is correct?
a. | Reaction I is faster because it has a higher
Ea . | c. | Reaction I is slower because it is
exothermic. | b. | Reaction II is
faster because it has a lower Ea . | d. | Reaction II is slower because it is
endothermic. |
|
|
|
5.
|
Consider the following reaction mechanism:
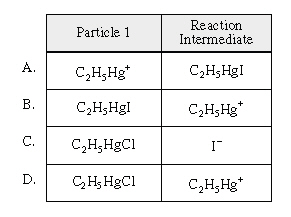
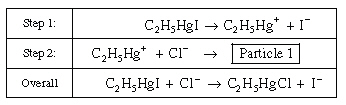 Identify Particle 1 and a reaction intermediate
from the above mechanism. Identify Particle 1 and a reaction intermediate
from the above mechanism.
|
|
|
6.
|
Due to a change in temperature, a system at
equilibrium shifts, causing the concentration
of products to change. Which of the following could
be correct?
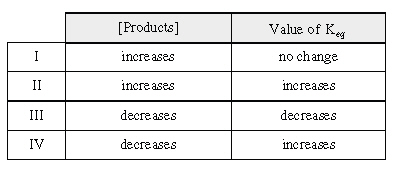
a. | I only | c. | I and IV only | b. | II only | d. | II and III only |
|
|
|
7.
|
Which of the following describes “activation energy”?
a. | the amount of energy that product molecules possesses | c. | the amount of energy released when
reactant molecules collide | b. | the difference between the Ep of the products
and the Ep of the reactants | d. | the minimum amount of energy required to start a chemical
reaction |
|
|
|
8.
|
If some O2
is injected into the equilibrium system below, which of the
following is correct?
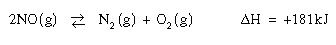
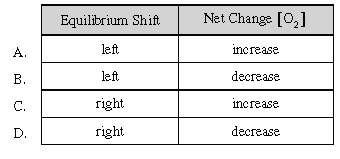
|
|
|
9.
|
Consider the equilibrium:
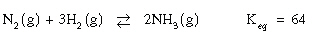 A 1.0L container is filled with 0.28 mol
N2 , 0.16 mol H2 and 0.54 mol NH3 . A 1.0L container is filled with 0.28 mol
N2 , 0.16 mol H2 and 0.54 mol NH3 .
In which direction will the reaction
proceed and what will happen to the pressure of the system?
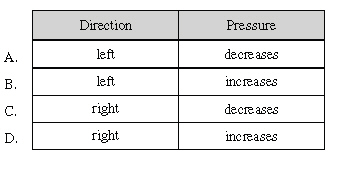
|
|
|
10.
|
Solid sodium metal reacts rapidly with water in an
open beaker to produce aqueous
sodium hydroxide and hydrogen gas. A change in which of the
following could be
used to measure the rate of this reaction?
a. | the volume of the solution | c. | the concentration of the solid sodium | b. | the pressure of the hydrogen gas | d. | the mass of the
beaker and its contents |
|